Gene Editing and Miniature Brain Clusters
02.10.2023 posted by Admin
Gene editing combined with the cultivation of miniature brain cell clusters in a lab setting is a powerful technique researchers are using to delve into the role of specific genes in brain development and disorders. This innovative approach has significant implications for our understanding of neurodevelopmental conditions, potentially paving the way for more targeted therapeutic interventions.
To grasp the significance of this research, it's important to recognize that mutations in numerous genes are linked to neurodevelopmental disorders, such as autism, schizophrenia, and epilepsy. However, the precise mechanisms through which these genes impact neuron function have remained largely elusive. Unraveling this mystery could provide valuable insights for therapies aimed at addressing these genes and the biological processes they influence.
The process operates as follows: During the brain's development, specialized cells known as interneurons assume distinct roles and migrate within the brain to establish circuits responsible for regulating various brain functions.
Scientists have conjectured that disruptions in the signaling balance between interneurons that either stimulate or inhibit the activity of other neurons within these circuits may contribute to the development of conditions like autism, schizophrenia, and epilepsy. This signaling imbalance could potentially serve as a common denominator underlying these diverse disorders.
In this groundbreaking study, led by Sergiu Pasca, a neurobiologist at Stanford University, and his team, the researchers harnessed CRISPR gene editing techniques and the ability to cultivate brain organoids to investigate the role of interneurons in these processes.
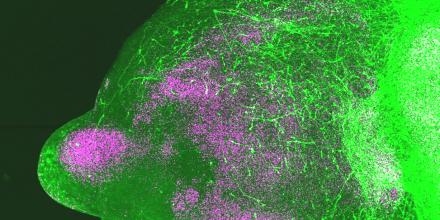
Building upon prior methodologies, the researchers grew stem cells into brain organoids representing the subpallium (a deep brain region where many inhibitory brain cells originate) and the cortex. When these organoids were combined, the cells exhibited movements mirroring their natural development in utero.
In their latest research, featured in a recent publication in Nature, the team created over a thousand subpallium and cortex organoids. They employed CRISPR gene editing to disrupt the function of 425 different genes linked to autism, each within its respective organoid. Subsequently, they observed how these neurons behaved using a fluorescent dye.
The findings were illuminating. The study revealed that approximately 10% of the more than 400 genes tested interfered with the generation and migration of interneurons during brain development. Some of these outcomes were unexpected, such as the discovery that a gene associated with a rare, severe childhood disease affected cell organelles and impeded cell migration in the brain.
Nonetheless, it's crucial to acknowledge certain limitations. There may be other stages of development where genes exert their influence, which this study did not investigate. Additionally, genes associated with rare neurodevelopmental disorders often do not act in isolation. Variations in how these genes manifest across individuals can lead to differing outcomes, such as autism, schizophrenia, or no apparent effects. Expanding the research to encompass a broader range of donors may help shed light on these variable effects.
Looking at the broader picture, the next phase of this research aims to conduct similar screenings for other brain processes that could be disrupted by disorders and diseases. These include how cells mature, differentiate into various types, and establish connections (synapses). Understanding these processes may offer a fresh perspective on psychiatric disorders, which are currently defined primarily by behavioral criteria rather than underlying biological mechanisms. In essence, this research opens the door to a new era of understanding and potentially treating complex neurological conditions.
To grasp the significance of this research, it's important to recognize that mutations in numerous genes are linked to neurodevelopmental disorders, such as autism, schizophrenia, and epilepsy. However, the precise mechanisms through which these genes impact neuron function have remained largely elusive. Unraveling this mystery could provide valuable insights for therapies aimed at addressing these genes and the biological processes they influence.
The process operates as follows: During the brain's development, specialized cells known as interneurons assume distinct roles and migrate within the brain to establish circuits responsible for regulating various brain functions.
Scientists have conjectured that disruptions in the signaling balance between interneurons that either stimulate or inhibit the activity of other neurons within these circuits may contribute to the development of conditions like autism, schizophrenia, and epilepsy. This signaling imbalance could potentially serve as a common denominator underlying these diverse disorders.
In this groundbreaking study, led by Sergiu Pasca, a neurobiologist at Stanford University, and his team, the researchers harnessed CRISPR gene editing techniques and the ability to cultivate brain organoids to investigate the role of interneurons in these processes.
Building upon prior methodologies, the researchers grew stem cells into brain organoids representing the subpallium (a deep brain region where many inhibitory brain cells originate) and the cortex. When these organoids were combined, the cells exhibited movements mirroring their natural development in utero.
In their latest research, featured in a recent publication in Nature, the team created over a thousand subpallium and cortex organoids. They employed CRISPR gene editing to disrupt the function of 425 different genes linked to autism, each within its respective organoid. Subsequently, they observed how these neurons behaved using a fluorescent dye.
The findings were illuminating. The study revealed that approximately 10% of the more than 400 genes tested interfered with the generation and migration of interneurons during brain development. Some of these outcomes were unexpected, such as the discovery that a gene associated with a rare, severe childhood disease affected cell organelles and impeded cell migration in the brain.
Nonetheless, it's crucial to acknowledge certain limitations. There may be other stages of development where genes exert their influence, which this study did not investigate. Additionally, genes associated with rare neurodevelopmental disorders often do not act in isolation. Variations in how these genes manifest across individuals can lead to differing outcomes, such as autism, schizophrenia, or no apparent effects. Expanding the research to encompass a broader range of donors may help shed light on these variable effects.
Looking at the broader picture, the next phase of this research aims to conduct similar screenings for other brain processes that could be disrupted by disorders and diseases. These include how cells mature, differentiate into various types, and establish connections (synapses). Understanding these processes may offer a fresh perspective on psychiatric disorders, which are currently defined primarily by behavioral criteria rather than underlying biological mechanisms. In essence, this research opens the door to a new era of understanding and potentially treating complex neurological conditions.